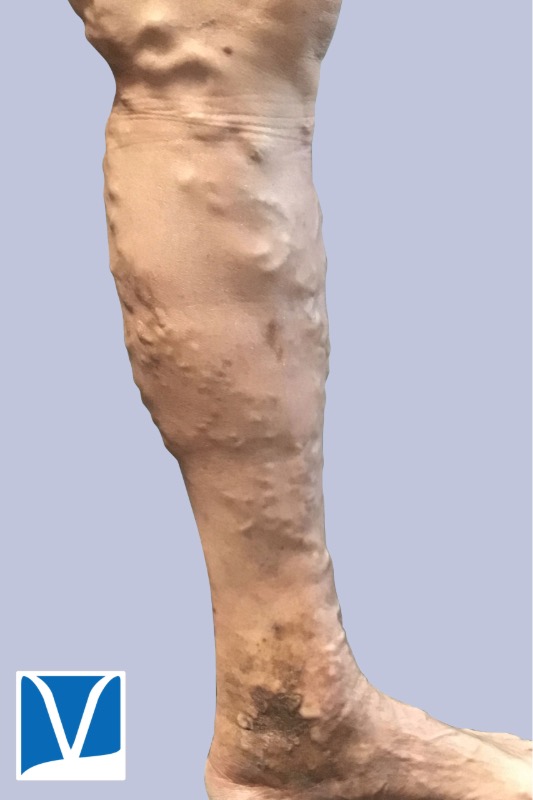
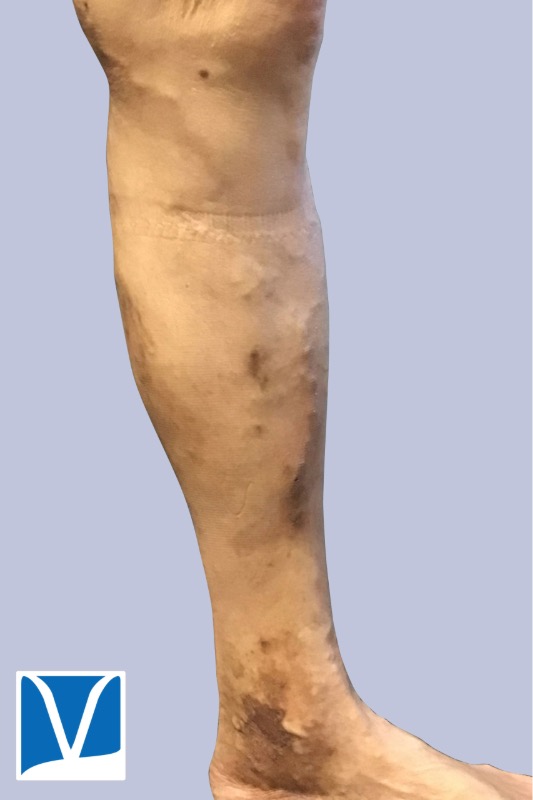
VenaSeal vein treatment is one of the newest advances in the treatment of varicose veins. VenaSeal is a medical grade adhesives administered using ultrasound guidance close off the saphenous veins, which are often the source of large branch varicose veins patients see on the surface of their legs. VenaSeal was FDA approved in 2015 with the indication for the permanent closure of lower extremity superficial truncal veins, such as the great saphenous vein (GSV).
The VenaSeal procedure is the only non-tumescent, non-thermal, non-sclerosant procedure approved for use in the U.S. that uses a specially formulated medical adhesive that closes the vein. This unique approach may eliminate the risk of nerve injury that is sometimes associated with certain thermal-based procedures.
The procedure is administered without the use of tumescent anesthesia, minimizing the need for multiple needle sticks. Patients also report minimal-to-no bruising post procedure and in some cases, do not have to wear a compression stocking after the procedure.
See How the Procedure is Perfomed
The VenaSeal procedure is an office based procedure performed with a single local injection of lidocaine in the skin. It uses a novel technique where a catheter is placed on the inside of the saphenous vein under ultrasound guidance, and advanced up the diseased section of the vein. A surgical adhesive similar to super glue is used to close the vein.
With this technique, tumescent anesthesia is not required, nor is compression stockings after the procedure. Because we are not using heat to close the vein, there is minimal risk of skin or nerve injury as a complication of the procedure.
Patients can return to work the day of the procedure, and return to regular activity a day or two after the procedure. One consideration is the possibility of an allergy to the glue, which is cyanoacrylate based. Thus, its important to discuss industrial glue exposure and other allergies when considering this procedure.
Clinical Trial Results
The VenaSeal procedure is shown to be safe and effective, with consistent results across three clinical trials. Closure rates in the first clinical trial were 92 percent at 12 and 24 months, respectively. Results from the European Sapheon Closure System Observational ProspectivE (eSCOPE) study published in the Journal of Vascular Surgery demonstrate a cumulative closure rate of 92.9 percent and improvement in quality of life scores at 12 months. Additionally, the three month, 12 month and 24 month results of the VeClose pivotal study published in the Journal of Vascular Surgery continue to demonstrate safety and efficacy of the VenaSeal procedure with excellent closure rates of 98.9 percent. As a result of these encouraging clinical trial results, CMS provided reimbursement to cover this procedure for medicare patients, and it is expected that many private health insurance companies will follow in the coming years. In these studies VenaSeal was compared to Closurefast. The main difference is that compared to Closurefast there is less need for needlesticks for anesthesia during the procedure, and thus patients report its more comfortable and they have less bruising in early recovery. Over time, however, the results are quite similar to Closurefast.
The Inovia VenaSeal Vein Treatment Experience
We were the first clinic in Oregon to perform VenaSeal. As reported in the Bend, Bulletin, in 2013, our first patient described the 20 minute procedure as painless, other than the needlestick to put in the local anesthetic: “It was maybe 30 seconds of pain and it was minimal. Then there was just a bit of discomfort because you can kind of sense some tugging as they go up your leg,” she said. “I can’t tell you how slick and easy it was.”
We went on to participate in the original VeClose pivitol trial where we enrolled the second largest number of patients in the trial. We are still following these patients 5 years later. As an investigator and author of the study, Dr. Jones has been invited to present this data and numerous national and international meetings. In addition, doctors have traveled from all over the country and world to observe how we do this procedure as part of their training to offer this exciting technology to their patients.
One of the advantages of coming to Inovia is that we offer nearly all of the latest, most innovative varicose vein treatments. We can assess your particular situation, offer you options, and let you decide which treatment is best for you.
You can find out if VenaSeal is right for you.
Used With Permission | Results May Vary
These Photos Are of an Actual Patient of Our Practice Who Has Provided Consent to Display Their Pictures Online. Keep in Mind That Each Patient is Unique and Results May Not Occur for All Patients. Please Book a Consultation for More Info on How Treatments May Help You.
At Inovia Vein Specialty Centers we are leading vein doctors who specialize in caring for patients with venous disorders such as varicose veins, deep venous thrombosis, and venous stasis ulcers. Founded in 2006, we have specialty vein clinics across the Pacific Northwest. Call to set up a consultation to learn more about your treatment options.
Frequently Asked Questions
VenaSeal
In most cases, patients can travel as soon as the day after a VenaSeal procedure. However, there are important considerations to take for long-term travel that may restrict leg movement.
VenaSeal is essentially a medical grade superglue known as cyanoacrylate. This type of glue has been used for many years for brain surgery, heart surgery, plastic surgery, trauma surgery and in other indications, such as to close cuts on children in the emergency room as an alternative to stitches.
Some swelling after a vein procedure is not unexpected or uncommon. Generally the compression stocking can help with this the first week. If there is any concern, its best to have it evaluated by a vein care provider.
Complications are uncommon. Rarely, allergies to the VenaSeal adhesive have been reported. Otherwise, the same risks as with other vein treatments exist - such as a small risk of blood clot, bleeding, infection or pain.
The night of the procedure we generally advise patients to go home, put their leg up on some pillows, and take it easy. In most cases, patients can go back to all their usual activities - including exercise - the next day.
With VenaSeal, the target vein being treated is sealed right away. Some patients start to feel an improvement in their symptoms by the next day, but others may not notice an improvement for several weeks and even months. It can take several months or longer for the branch varicose veins on the surface to fade from view.
VenaSeal is an FDA approved medical grade adhesive that can be used to seal the saphenous veins to help treat venous insufficiency. In some cases, an inflammatory reaction can form to the treatment leading to a red streak over the vein. This generally fades over a few weeks. Rarely, allergies to the adhesive have been reported.